Test Amit sep
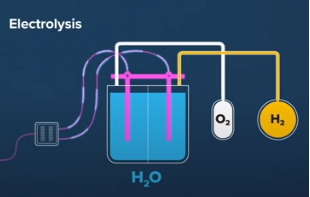
Electrolyzer: A device that utilizes electricity to split water into hydrogen and oxygen
- Alkaline Electrolyzer: This type of electrolyzer uses an aqueous solution of potassium hydroxide (KOH) as the electrolyte, and it is commonly used for industrial-scale hydrogen production.
- Proton Exchange Membrane (PEM) Electrolyzer: This type of electrolyzer uses a polymer membrane as the electrolyte, and it is widely used for producing hydrogen for fuel cell vehicles and stationary applications.
- An Anion Exchange Membrane (AEM) Electrolyzer is a type of electrolyzer that uses a membrane that allows the flow of negatively charged ions, or anions, while preventing the flow of cations.
- Solid Oxide Electrolyzer: This type of electrolyzer uses a solid oxide material as the electrolyte, and it is capable of producing hydrogen at high temperatures and pressures.
- Bioelectrolyzer: This type of electrolyzer uses microorganisms, such as bacteria, to produce hydrogen through biological processes.
- Lead-Acid Electrolyzer: This type of electrolyzer uses lead-acid batteries as the energy source for the electrolysis process, and it is commonly used for small-scale hydrogen production.
An electrolyzer is a device that utilizes electricity to split water into hydrogen (H2) and oxygen (O2). The process is called electrolysis, and it is the reverse of the reaction that occurs in a fuel cell, where hydrogen and oxygen are combined to produce water and electricity. Electrolyzers are used in various applications, including hydrogen fuel production, metal plating, and water purification.
Water splitting is the process of separating water into its components, hydrogen, and oxygen, through the application of electrical energy. This is done through a process called electrolysis, where an electric current is passed through an aqueous solution of water. The positively charged ions, or cations, are attracted to the cathode, while the negatively charged ions, or anions, are attracted to the anode. As a result, hydrogen gas is generated at the cathode, and oxygen gas is generated at the anode. This process can be used for various purposes, including the production of hydrogen fuel, the purification of water, and the production of oxygen for medical and industrial use.
There are several types of electrolyzers, including:
- Alkaline Electrolyzer: This type of electrolyzer uses an aqueous solution of potassium hydroxide (KOH) as the electrolyte, and it is commonly used for industrial-scale hydrogen production.
- Proton Exchange Membrane (PEM) Electrolyzer: This type of electrolyzer uses a polymer membrane as the electrolyte, and it is widely used for producing hydrogen for fuel cell vehicles and stationary applications.
- An Anion Exchange Membrane (AEM) Electrolyzer is a type of electrolyzer that uses a membrane that allows the flow of negatively charged ions, or anions, while preventing the flow of cations.
- Solid Oxide Electrolyzer: This type of electrolyzer uses a solid oxide material as the electrolyte, and it is capable of producing hydrogen at high temperatures and pressures.
- Bioelectrolyzer: This type of electrolyzer uses microorganisms, such as bacteria, to produce hydrogen through biological processes.
- Lead-Acid Electrolyzer: This type of electrolyzer uses lead-acid batteries as the energy source for the electrolysis process, and it is commonly used for small-scale hydrogen production.
Each type of electrolyzer has its own advantages and disadvantages, and the choice of electrolyzer depends on the specific application and the desired outcome.
An Alkaline Electrolyzer is a type of electrolyzer that uses an aqueous solution of potassium hydroxide (KOH) as the electrolyte. It is commonly used for industrial-scale hydrogen production, and it is one of the oldest and most established types of electrolyzers. Alkaline electrolyzers have a number of advantages, including high hydrogen production efficiency, low cost, and long service life. They are also well-suited for large-scale hydrogen production, as they can handle high flow rates of water and electricity, and they are relatively simple and robust in design. On the other hand, alkaline electrolyzers require careful handling of the potassium hydroxide electrolyte, as it is highly caustic and can cause skin irritation or chemical burns. Additionally, the aqueous solution can corrode certain metals and materials, which can reduce the service life of the equipment.
A Proton Exchange Membrane (PEM) Electrolyzer is a type of electrolyzer that uses a polymer membrane as the electrolyte. The membrane acts as a barrier that allows only positively charged ions, or protons, to pass through while preventing the flow of electrons. PEM electrolyzers are widely used for producing hydrogen for fuel cell vehicles and stationary applications due to their high efficiency, compact size, and rapid response times. They are typically designed to operate at low temperatures and pressures, making them well-suited for use in portable and mobile applications. Additionally, PEM electrolyzers produce pure hydrogen gas with very little contamination, making them ideal for fuel cell systems requiring high-purity hydrogen. On the other hand, PEM electrolyzers are relatively expensive compared to other types of electrolyzers, and they require careful maintenance of the polymer membrane to ensure long-term performance and reliability.
An Anion Exchange Membrane (AEM) Electrolyzer is a type of electrolyzer that uses a membrane that allows the flow of negatively charged ions, or anions, while preventing the flow of cations. Unlike Proton Exchange Membrane (PEM) electrolyzers, which are optimized for hydrogen production, AEM electrolyzers are designed to produce oxygen. They work by transporting oxygen ions from the anode to the cathode through the AEM, while preventing the migration of hydrogen ions. AEM electrolyzers have the potential to offer improved performance and efficiency compared to traditional oxygen-producing electrolyzers, such as alkaline electrolyzers. However, they are a relatively new technology and further development and optimization are needed to make them practical and cost-effective for widespread commercial use.
A Solid Oxide Electrolyzer (SOE) is a type of electrolyzer that uses a solid oxide material, such as cerium oxide (CeO2), as the electrolyte. It is capable of producing hydrogen at high temperatures and pressures, and it is considered a promising technology for large-scale hydrogen production. One of the key advantages of SOEs is that they can operate at elevated temperatures, which allows for improved energy efficiency and reduced costs. Additionally, SOEs are capable of utilizing a variety of feedstocks, including natural gas, biogas, and other hydrogen-rich gases, which makes them flexible and versatile. On the other hand, SOEs are relatively complex and require a high-temperature operation, which can result in technical challenges and increased costs. Additionally, they are not yet widely commercialized, and further development and optimization is needed to make them practical and cost-effective for widespread use.
A Bioelectrolyzer is a type of electrolyzer that uses microorganisms, such as bacteria, to produce hydrogen through biological processes. It operates by applying an electrical current to a solution containing hydrogen-producing microorganisms, which causes the organisms to produce hydrogen gas. Bioelectrolyzers have the potential to offer a sustainable and environmentally friendly approach to hydrogen production, as they do not rely on fossil fuels and can utilize waste streams as a source of energy. Additionally, bioelectrolyzers can operate at ambient temperatures and pressures, which reduces energy requirements and costs compared to other types of electrolyzers. However, bioelectrolyzers are still a relatively new technology, and further development and optimization is needed to improve the efficiency and scalability of the process. Additionally, the hydrogen production rate and purity of bioelectrolyzers can be limited, and the microorganisms used in the process can be sensitive to environmental conditions, which can affect their performance and stability.
A Lead-Acid Electrolyzer is a type of electrolyzer that uses lead-acid batteries as the source of electricity. The batteries provide the electrical energy needed to split water into hydrogen and oxygen, and the hydrogen produced can be stored and used as fuel. Lead-Acid Electrolyzers are relatively simple and inexpensive, and they can be easily adapted for use in remote and off-grid locations. However, they have several disadvantages, including low efficiency, short battery life, and the need for regular maintenance and replacement of the batteries. Additionally, lead-acid batteries are considered environmentally harmful, as they contain lead, a toxic heavy metal, and they can pose a risk to human health and the environment if not handled properly. Lead-Acid Electrolyzers are not widely used and have largely been superseded by more efficient and environmentally friendly technologies.
How would you rate this Article ?
Press the number of stars to rate this Magazine.
4.5 Author Points.
Based on 20 Magazines written by this author.
No Magazine Ratings yet


























Please Sign In or Sign Up to leave a Comment.